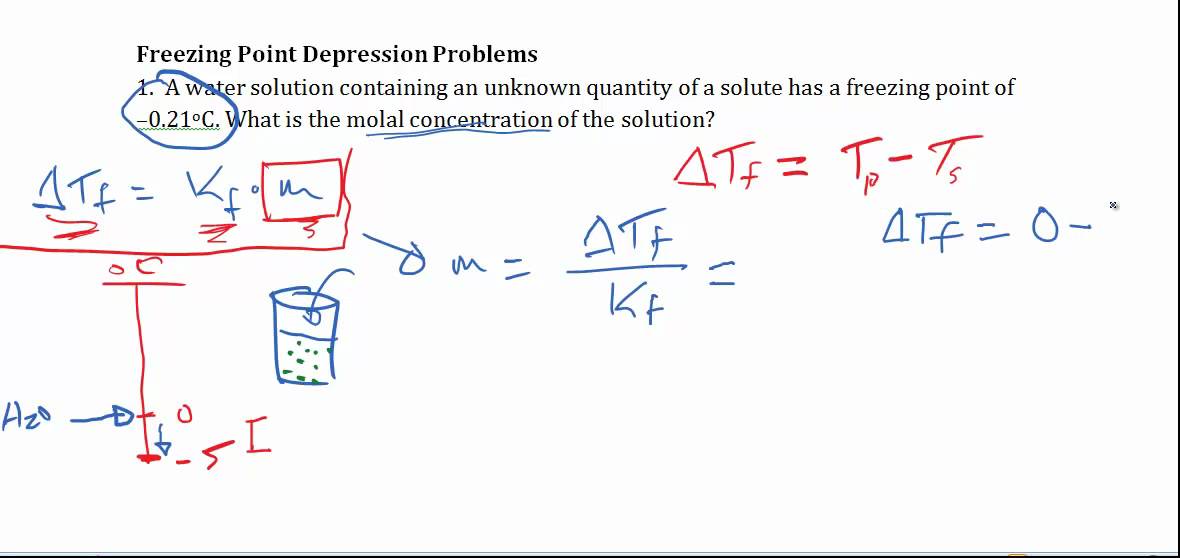
Depression Of Freezing Point . By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. It is a colligative property of solutions that is generally proportional to the molality of the added solute. Freezing point depression is a colligative property observed in solutions that results from the introduction of. Freezing point depression occurs when the freezing point of a liquid is lowered or depressed by adding another compound to it. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes.
from www.youtube.com
The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. Freezing point depression is a colligative property observed in solutions that results from the introduction of. Freezing point depression occurs when the freezing point of a liquid is lowered or depressed by adding another compound to it. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. It is a colligative property of solutions that is generally proportional to the molality of the added solute. By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes.
calculating freezing point depression YouTube
Depression Of Freezing Point Freezing point depression is a colligative property observed in solutions that results from the introduction of. It is a colligative property of solutions that is generally proportional to the molality of the added solute. Freezing point depression is a colligative property observed in solutions that results from the introduction of. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. Freezing point depression occurs when the freezing point of a liquid is lowered or depressed by adding another compound to it. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes.
From thechemistrynotes.com
Depression of Freezing Point Depression Of Freezing Point It is a colligative property of solutions that is generally proportional to the molality of the added solute. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. Freezing point depression occurs when the freezing point of a liquid is lowered or depressed by adding another compound to. Depression Of Freezing Point.
From www.jove.com
11369.jpg Depression Of Freezing Point It is a colligative property of solutions that is generally proportional to the molality of the added solute. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the.. Depression Of Freezing Point.
From www.chemistrynotmystery.com
Colligative properties Depression in freezing point Chemistry!!! Not Depression Of Freezing Point By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and. Depression Of Freezing Point.
From www.thoughtco.com
What Freezing Point Depression Is and How It Works Depression Of Freezing Point The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of. Depression Of Freezing Point.
From www.slideserve.com
PPT Solutions part 2 PowerPoint Presentation ID706322 Depression Of Freezing Point Freezing point depression occurs when the freezing point of a liquid is lowered or depressed by adding another compound to it. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the. Depression Of Freezing Point.
From www.youtube.com
Freezing Point Depression Problems & Example (Colligative Property Depression Of Freezing Point By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. Freezing point depression is a colligative property observed in solutions that results from the introduction of. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. The freezing point depression is the. Depression Of Freezing Point.
From www.slideserve.com
PPT Determining the Molecular Mass by Freezing Point Depression Depression Of Freezing Point By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution.. Depression Of Freezing Point.
From www.chegg.com
2. Freezing Point Depression and Activit You can use Depression Of Freezing Point It is a colligative property of solutions that is generally proportional to the molality of the added solute. By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. Freezing point depression is a colligative property observed in solutions that results from the introduction of. Freezing point depression refers to the. Depression Of Freezing Point.
From facts.net
12 Extraordinary Facts About Freezing Point Depression Depression Of Freezing Point The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and. Depression Of Freezing Point.
From youtube.com
Calculate Freezing Point Depression YouTube Depression Of Freezing Point It is a colligative property of solutions that is generally proportional to the molality of the added solute. By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution.. Depression Of Freezing Point.
From www.youtube.com
Freezingpoint Depression Intro & Theory YouTube Depression Of Freezing Point By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. Freezing point depression occurs when the freezing point of a liquid is lowered or depressed by adding another. Depression Of Freezing Point.
From www.youtube.com
depression of freezing point solutions solutions class 12 chemistry Depression Of Freezing Point It is a colligative property of solutions that is generally proportional to the molality of the added solute. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of. Depression Of Freezing Point.
From www.youtube.com
calculating freezing point depression YouTube Depression Of Freezing Point Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. Freezing point depression occurs when the freezing point of a liquid is lowered or depressed by adding another compound to it. It is a colligative property of solutions that is generally proportional to the molality of the added solute. By analogy to. Depression Of Freezing Point.
From www.youtube.com
Freezing Point Depression Example YouTube Depression Of Freezing Point Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. Freezing point depression occurs when the freezing point of a liquid is lowered or depressed by adding another compound to it. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the. Depression Of Freezing Point.
From www.youtube.com
Boiling Point Elevation and Freezing Point depression YouTube Depression Of Freezing Point Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. It is a colligative property of solutions that is generally proportional to the molality of the added solute. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. Freezing point. Depression Of Freezing Point.
From www.youtube.com
Depression in freezing point,UNIT2, Class12. YouTube Depression Of Freezing Point Freezing point depression occurs when the freezing point of a liquid is lowered or depressed by adding another compound to it. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. It. Depression Of Freezing Point.
From general.chemistrysteps.com
Freezing Point Depression Chemistry Steps Depression Of Freezing Point The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and. Depression Of Freezing Point.
From www.thoughtco.com
How to Calculate Freezing Point Depression Depression Of Freezing Point The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. By analogy to our treatment of boiling point elevation,the freezing point depression (\(δt_f\)) is defined as the difference between the. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and. Depression Of Freezing Point.